Abstract
Subcutaneous Apomorphine Infusion Effects on OFF Time and Motor Symptoms in Individuals with Parkinson’s Disease: A Systematic Review and Meta-Analysis
Pages: 3-16
Category: Review
Published Date: 30-07-2025
Paula Abola 1,*, Benjamin Wolden 1 and Mitchell Wolden1
Author Affiliation:
Department of Clinical Research, University of Jamestown, USA
Keywords:
Parkinson’s Disease, dyskinesia, motor symptoms
Abstract:
Background: Parkinson’s Disease (PD) is a progressive neurodegenerative disorder characterized by motor fluctuations and dyskinesias that become increasingly challenging to manage in advanced stages. Continuous subcutaneous Apomorphine infusion offers a less invasive alternative to device-aided therapies such as deep brain stimulation and Levodopa-Carbidopa intestinal gel. This systematic review and meta-analysis aimed to evaluate the efficacy of Apomorphine infusion in reducing OFF time and motor symptom severity, focusing on Unified Parkinson’s Disease Rating Scale (UPDRS) Part III scores in individuals with advanced PD.
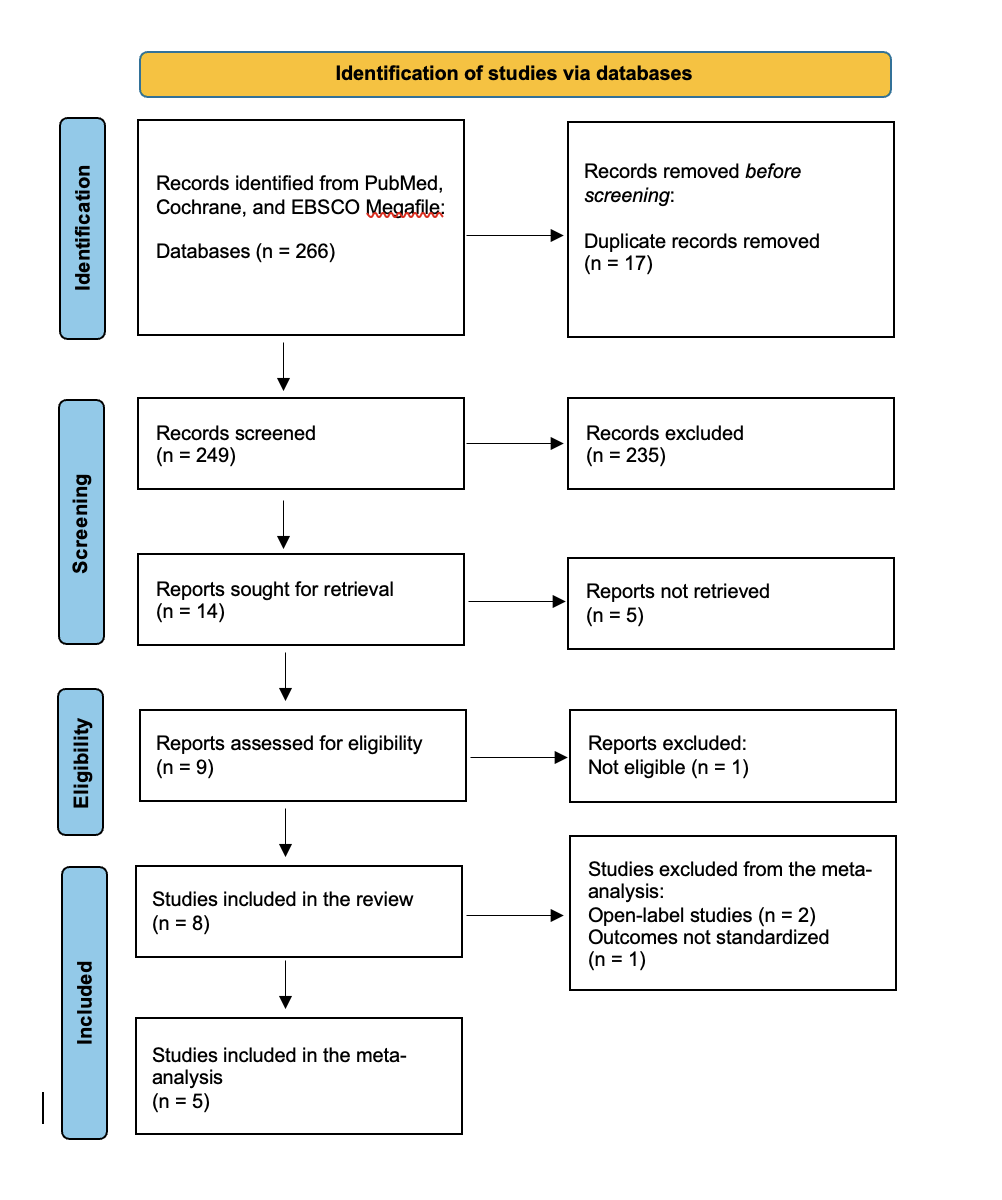
Objectives: A systematic search of PubMed, Cochrane, and EBSCO Megafile databases was conducted through April 16, 2025, according to PRISMA guidelines. Randomized controlled trials (RCTs) evaluating Apomorphine infusion and reporting outcomes on OFF time and UPDRS Part III were included. Risk of bias was assessed using the Cochrane RoB 2 tool, and certainty of evidence was evaluated with GRADE. Meta-analyses were performed using a random effects model.
Result: Eight studies (n = 458) met the inclusion criteria, of which five were eligible for meta-analysis. Apomorphine infusion significantly reduced OFF time compared to placebo (MD = -1.93 hours; 95\% CI: -2.91 to -0.95; low-certainty evidence), with minimal heterogeneity (I² = 0\%). A significant reduction was also observed in UPDRS Part III scores (MD = -19.11; 95\% CI: -25.54 to -12.68; very low-certainty evidence), although substantial heterogeneity was present (I² = 67.93\%).
Conclusion: This systematic review supports the efficacy of Apomorphine infusion in reducing OFF time and improving motor symptoms in individuals with advanced PD. Apomorphine infusion represents a treatment option, particularly for patient’s ineligible for surgical interventions. However, the overall certainty of evidence is limited by methodological heterogeneity and a small number of high-quality trials. Future studies should aim for standardized outcome measures, long-term comparisons with other device-aided therapies, and exploration