Full HTML
Immunoadsorption vs. Plasma Exchange in Guillain-Barre Syndrome: A Meta-Analysis and Systematic Review - Volume 1 Issue 1 , - (6 Months )
Pages: 8-16
Category: Original Research
Published Date: 09-05-2024
Levin Ace Danganan1*, Elizer Montemayor1, Renato Dejan Jr.1
Author Affiliation:
Department of Neurosciences, East Avenue Medical Center, Philippines.
Keywords:
Immunoadsorption, Plasma Exchange, Therapeutic Apheresis, Guillain-Barre Syndrome
Full Text:
ABSTRACT
Background: Guillain-Barre Syndrome is a debilitating neurological disease with an incidence of 1.1 - 1.8 per 100,000. Autoantibodies affecting peripheral nerve membranes play an important role in understanding the pathophysiology and treatment of it. Treatment options for its cure continue to unfold and evolve. Different clinical trials resulted in increased interest in therapeutic apheresis for treatment of severe and refractory disease.
Objectives: Conflicting results of immunoadsorption compared to plasma exchange in the management of Guillain-Barre Syndrome led us to synthesize available evidence from published studies.
METHODS: Review Manager software was used for this review and classified the outcomes into primary (curative effect) and secondary (safety profile and relapse rate). Quality assessment and statistical data analysis were conducted using the said software.
Result: The odds of achieving at least one grade disability and functional improvement was similar for patients treated with immunoadsorption and plasma exchange (OR: 0.77; 95% CI: 0.34 - 1.74; p = 0.53). Reduced risk of complications for patients treated with immunoadsorption group as compared to plasma exchange group (RR: 0.69; 95% CI: 0.43 - 1.11; p = 0.13) was noted. Increased risk of relapse for patients who underwent immunoadsorption (RR: 1.70; 95% CI: 0.96 - 3.00; p = 0.07).
Conclusion: Immunoadsorption is at least as effective as plasma exchange in the treatment of Guillain-Barre Syndrome based on its curative effect by lowering its disability and improving functional score. Immunoadsorption showed reduced complications but relapse rates were higher compared to plasma exchange.
1. Introduction
The overall incidence of Guillain-Barre Syndrome’s (GBS) ranges from 1.1 - 1.8 cases per 100, 000 worldwide. The proposed mechanism of this debilitating neurological disease is that it is triggered by antigenic stimuli from viral or bacterial infections, or even vaccines manifesting as an ascending paralysis, numbness, and areflexia. It affects people of all age groups which is more common in males than females [1, 2]. Anti-gangliosides antibodies that affect peripheral nerve membranes are produced by molecular mimicry which plays an important role in understanding the pathophysiology and even the treatment of GBS [3].
Currently, standard treatments for GBS include Intravenous Immunoglobulin (IVIG), immunosuppressive Agents, Plasma Exchange (PE), and Immunoadsorption (IA) [4]. A recent study showed that IVIG and PE provide similar curative effects and relapse rates with reduced hospital and ventilation length [5]. Data from clinical trials resulted in increased interest in therapeutic apheresis for the treatment of severe and refractory autoimmune neurologic diseases.
PE is increasingly replaced by IA for escalating immunomodulatory treatments for GBS [6]. Several Randomized Controlled Trials (RCT) concluded that IA is at least as effective as PE in shortening the time from first symptom to first improvement and hospital duration [7, 8]. This is in contrast with another RCT wherein it showed later and slower improvement in disability in patients who underwent IA [9]. Due to these contradicting results, we conducted this meta- analysis.
and systematic review to synthesize available evidence from published studies to understand the effectiveness and safety profile of IA compared to PE in the management of Guillain-Barre Syndrome.
2. Methods
2.1. Protocol and Registration
We constructed this meta-analysis and systematic review following and observing the Cochrane collaboration and results were reported per the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. The protocol was not registered in any database.
2.2. Literature Search and Eligibility Criteria
Studies were identified by searching PubMed, Embase, Cochrane, and Google Scholar databases using the following search terms: (Immunoadsorption OR Hemoperfusion OR Tryptophan Adsorption OR Blood purification OR Blood filtration) AND (plasma exchange OR plasmapheresis) AND (Guillain- Barre? syndrome OR Acute inflammatory demyelinating polyneuropathy OR Miller Fisher syndrome OR Acute Motor-Sensory Axonal Neuropathy OR Acute Motor Axonal Neuropathy OR Landry’s Disease OR Ascending Paralysis). Consequently thereafter, we included articles that were written in English, directly comparing IA to PE in patients with GBS or its variants. In terms of the minimum date of publications, due to limited studies in the management of GBS comparing IA and PE, we included all relevant RCTs with more than 10 participants in each treatment group. Articles that did not meet these criteria or were animal and human model studies, systematic reviews, abstracts, letters to the editors, and case reports were excluded.
2.3. Data Extraction and Analysis
We extracted the following date and assessed each article independently: Author ID (First author's last name and publication year), participants' characteristics (sample size, age and gender), dosages of the interventions, and outcomes analyzed in each study (Table 1).
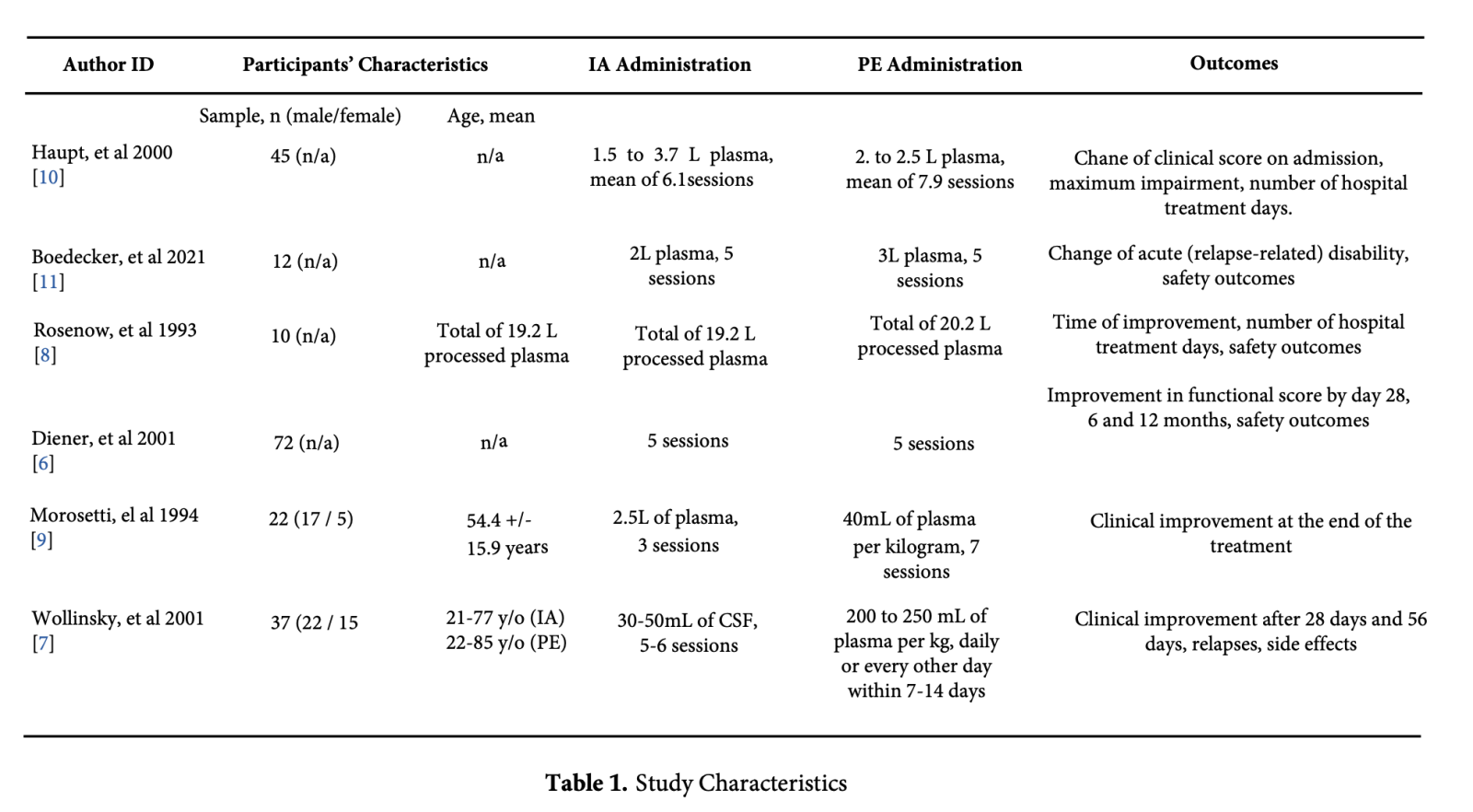
Afterward, we jointly carried out meta-analyses and systematic review using the Review Manager software (RevMan 5.4.1), and classified the outcomes into primary (i.e., the curative effect of IA and PE) and secondary (i.e., safety profile relating to complications of the treatment, and relapse rate). The rate of patients achieving improvement of ≥1 grades of disability scale four weeks after the treatment of GBS, the proportion of patients being able to walk unaided [7, 8], and at least a one grade improvement in the functional score by day 28 [5, 6] was the curative effect for this analysis.
A simple Odds Ratio (OR) was used for the calculations of the overall curative effect as the primary outcome, while the Risk Ratio (RR) was used for the secondary outcomes. Heterogeneity was also calculated using the I2 statistics, of which values ranging between 0 and 50%, 51–70%, and above 70% were considered low, moderate, and high, respectively.
2.4. Quality Assessment
Using the Cochrane's risk of bias tool provided in the RevMan version 5.4.1 and was based on the following elements namely selection, performance, attrition, detection, and reporting bias that evaluated the overall quality of each study (Figure 1).
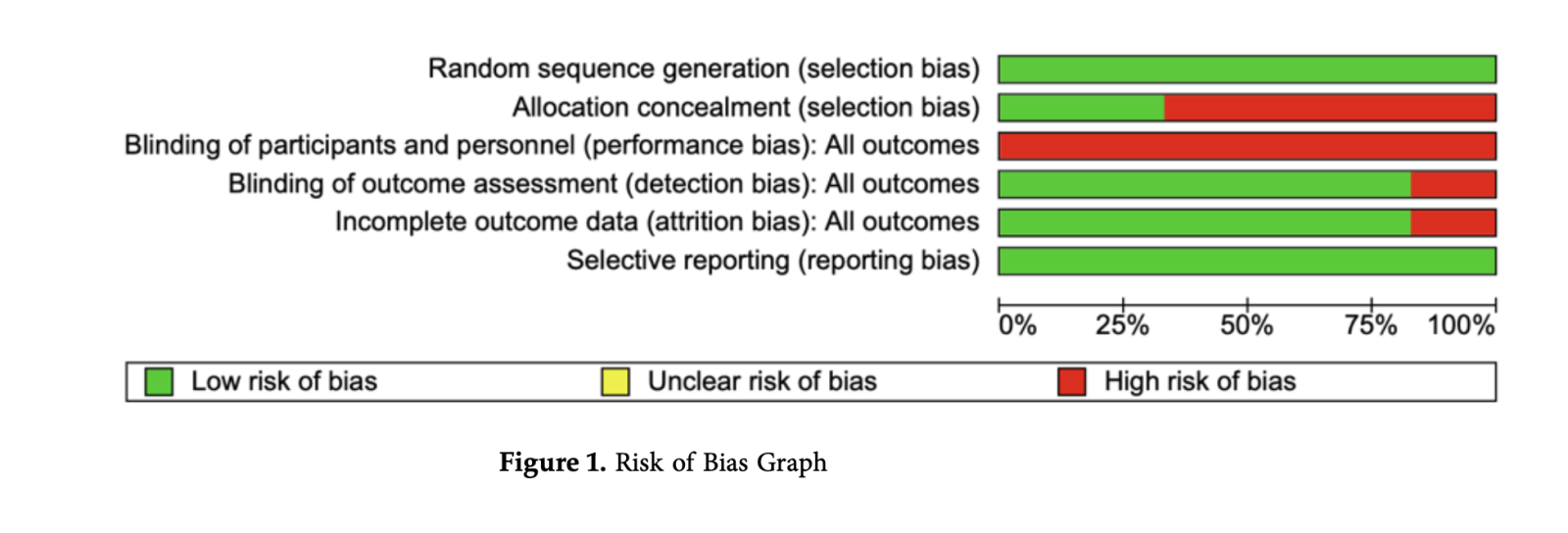
Good quality was assigned when all the assessment criteria were met while poor quality was assigned when two or more assessment criteria were of high risk of bias. Finally, fair quality was assigned when only one criterion was not met or two criteria had unclear risk of bias. Inadequate allocation concealment of the RCTs included in this meta-analysis and systematic review allows bias to seep in since there’s no mentioned evidence on how the different studies prevented the participants to know what treatment group they belong to. Likewise to blinding of participants and personnel, no mentioned techniques to avoid performance bias, this could affect the outcome of the study since the experimental group (IA) could have received more attention.
3. Result
3.1. Search Results
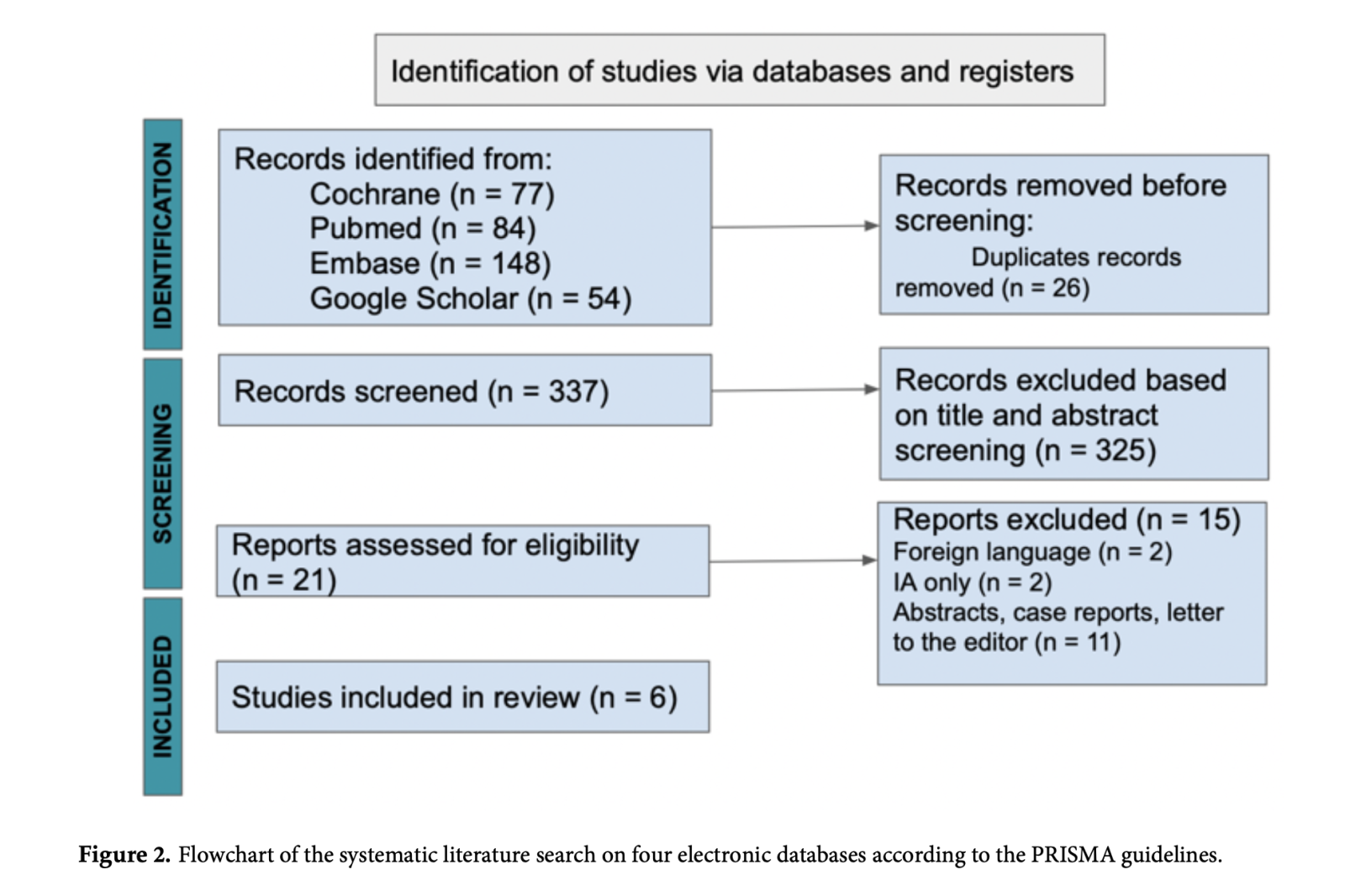
A total of 363 articles were identified after a detailed search through the electronic databases. We identified 26 duplicate articles, which were excluded from the review. Thereafter, the remaining 337 articles had their titles and abstracts screened, of which only 21 met the screening criteria. The said number of articles were assessed for eligibility, languages other than English, systematic reviews, case reports, letters to the editor, studies not comparing PE vs IA, and studies focusing on IA only were excluded. The selection results are shown using the PRISMA flow diagram (Figure 2).
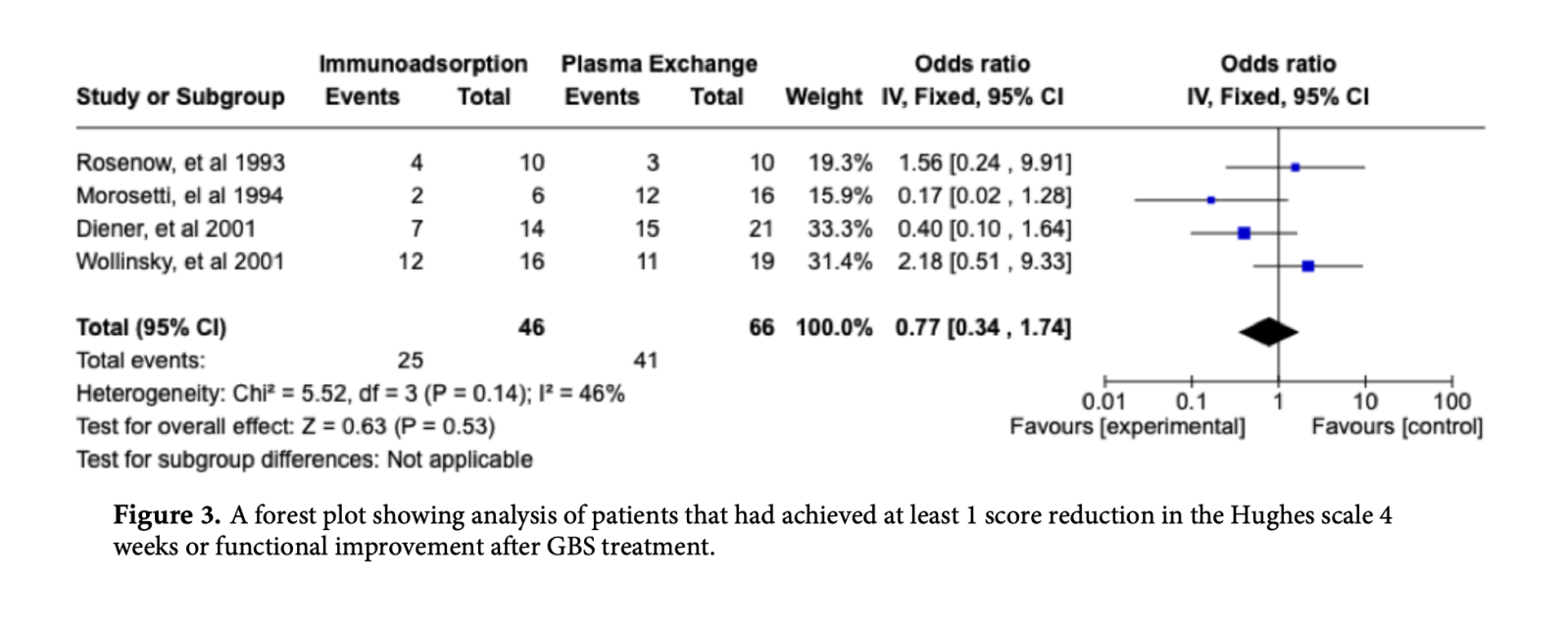
3.2. Primary Outcome: Curative Effect
The curative effect of IA and PE was assessed by identifying the number of patients that had achieved at least 1 score reduction in the Hughes scale 4 weeks after GBS treatment or with at least 1 grade improvement of functional score or those who were cured or those who were able to walk without support. The pooled results shown in below implicating the odds of achieving at least one grade disability or functional improvement was similar for patients treated with IA and PE was not statistically significant (OR: 0.77; 95% CI: 0.34 - 1.74; p = 0.53). (Figure 3).
The interval between the onset of neurological symptoms and a one grade improvement in the functional score as a measure of speed of recovery and duration of hospital stay were not statistically significant for both IA and PE treatment [7, 9]. The severity of disease when discharged home was nearly the same in all groups, hence the average time from first treatment to first improvement was 5.3 days after PE and 3.6 days after IA which was not statistically significant (Table 2).

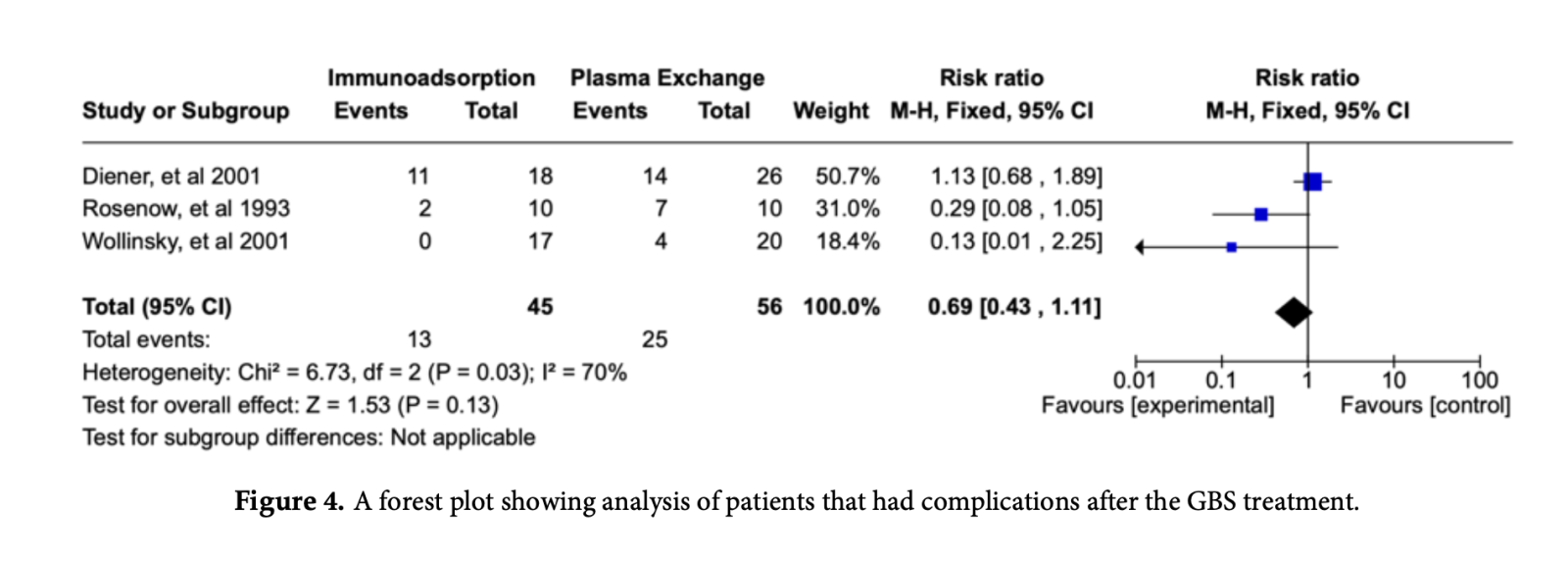
3.3.1. Secondary outcome: Safety profile
Safety profile was assessed with the number of patients who had allergic reactions,
hypotension, and catheter-related thrombosis in both IE and PE groups. For the safety profile of IA and PE, the pooled results showed the reduced risk of complications for patients treated with IA group as compared to PE group (RR: 0.69; 95% CI: 0.43 - 1.11; p = 0.13) but was not statistically significant (Figure 4).
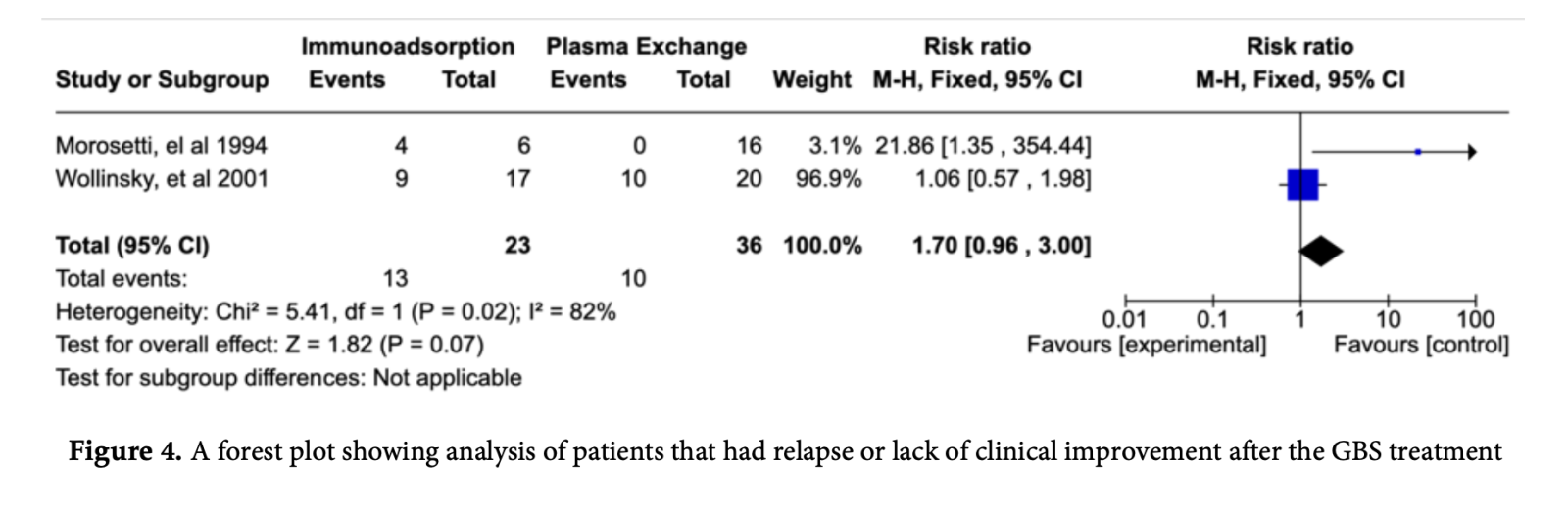
3.3.2. Secondary Outcome: Relapse
Lack of improvement, any relapse, or deteriorating disability score after IA and PE for the
treatment of GBS showed increased risk for patients underwent IA as shown by the pooled results (RR: 1.70; 95% CI: 0.96 - 3.00; p = 0.07) is not statistically significant (Figure 5).
4. Discussion
Treatment options for the cure of GBS continue to unfold and evolve. Moreover, the results of our study suggested that the curative effect and clinical improvement of patients with GBS is similar for both PE and IA, with a noted good safety profile but with increased risk of relapse for IA.
Clinical improvement for IA may be explained by the significant reduction of the pro- inflammatory cytokines IL-12, lL-17, IL-6, INF-γ, and TNF-α during treatment while the reverse is true for the PE treatment group [12]. The proposed mechanism is by changes of the cytokine serum concentration, IA and PE might influence cellular immunity. The lack of reduction in cytokines during treatment with PE could be a possible activation of cytokine production by the foreign fresh frozen plasma or albumin leading to activation of the immune system explaining the favorable clinical response in IA treatment [13]. Hence, selective IA is increasingly replacing PE due to its increasing knowledge on pathogenic relevance of autoantibodies [12].
Our meta-analysis has reported the statistically insignificance of the curative effect and clinical improvement of both treatments for GBS. The findings implicated that IA is at least as effective as PE in the management of GBS. It was found out that improvement of Hughes grade 4.2 ± 0.7 to 3.4 ± 0.9 (P < 0.001) occurred within 14.6 ± 15.5 days for patients treated with IA [12]. IA treatment as seen in Table 2 shows the different protocol used per controlled trials, it is said that 5 treatments over a period of 8-10 days with plasma treatment volumes 1 plasma volume seem to be an appropriate regimen to restore neurologic function. The efficacy of therapeutic apheresis be it PE or IA on autoimmune neurologic disorders such as GBS is based on immediate antibody elimination, pulsed induction of antibody redistribution, and immunomodulation.
Electrophysiologic studies in GBS would show the presence of abundant fibrillation and serious reduction in Motor Action Potential (MAP). To support the claim of effectiveness of the different treatment groups, electrophysiologic studies showed that Motor Conduction Velocities (MCV) were noted to have slight and unclear improvement in the PE treatment group, while no significant variation was noted in the IA treatment group after 45 days of treatment. It was recommended to study all sections of the nerve to see for reduction in MAP [8].
Based on the studies that were included in this meta-analysis and systematic review, two studies did not yield statistically significant findings about the hospitalization length of stay for the treatment of GBS by IA or PE. Conflicts of findings were also noted such that IA treatment group in one study showed 40% decreased in the hospital stay as compared to PE (25%) [7], while for the other showed slight clinical improvement was reported in 2 patients 15 days after the treatment with IA, prolonging their hospital stay [8]. With such a low number of evaluable patients and different outcomes per study protocol, evaluation for comparison is limited.
An important outcome that must be considered when comparing any treatment groups in a clinical trial are efficacy and safety. The risk ratio that our study yielded was less than 1, this implies that the secondary outcome for IA which is its safety profile has reduced risk, but statistically speaking, it is not significant as compared to PE (p = 0.27). Several studies reported that the allergic reactions such as urticaria and paresthesia, hypotension, and symptomatic hypocalcemia were noted more on the PE treatment group than IA [7,13]. Due to its superior safety profile, IA can be a therapeutic option for GBS if we are considering both the beneficial curative effect and low risk of complications [12].
Different studies suggested that therapeutic apheresis was used in patients with an autoimmune neurologic disease that is refractory to IVIG and/or immunosuppressants. Despite the use of both IA and PE, our meta-analysis showed that relapse or lack of improvement after 28 days of hospitalization were still noted, but more common in the IA treatment group as compared to PE with a risk ratio of more than 1 implying increased risk for the outcome that we sought which is relapse but not statistically significant. With such a low number of evaluable patients in the IA treatment group with a high disability score (grade 4 is bed bound and grade 5 is needing mechanical ventilation) at baseline, larger randomized controlled trials must be carried out to evaluate the relapse rate or lack of improvement for both treatment groups. Another motion to consider is about chronic IA as part of the long-term treatment of patients with GBS and other autoimmune neurologic diseases [12].
There are several limitations for this study including high heterogeneity that might threaten generalizability of our findings in the analysis of secondary outcomes such as the safety profile and relapse. This is expected since there are few studies being compared with varying sample sizes. Publication bias cannot be made for this study since we only included 6 studies following the systematic literature search. Another limitation is the omission of other studies written in foreign language that might strengthen the scientific research and might improve the statistical power of the meta-analyses.
5. Conclusion
There is still little evidence supporting the findings of the different RCTs included in this study. This meta-analysis and systematic review suggests that IA is at least as effective as PE in the
treatment of GBS based on its curative effect by improving its disability and functional score. With regards to safety profile, IA showed reduced complications as compared to PE, however relapse rates or lack of clinical improvement were higher in IA. IA is increasingly replacing PE due to its increasing knowledge on pathogenic relevance of autoantibodies and safety but health care professionals together with their patients should discuss a treatment regimen that is suitable to the patient and available for use.
Author Contributions: The research project was conceived, organized, and executed by Danganan LA,. who served as the main author of the study. Danganan LA conducted the literature search, data extraction, and analysis. Additionally, he was responsible for the design and execution of the statistical analysis. Furthermore, Danganan LA took the lead in manuscript preparation, contributing to the writing of both the initial draft and the final version. Montemayor E, acted as a co-author in the study and also participated in the conception, organization, and execution of the research project. He contributed to the literature search, data extraction, and analysis, providing valuable insights throughout the process. Dejan R, served as an adviser and co-author in the study and played a crucial role in manuscript preparation, contributing to the writing of both the initial and final drafts. Additionally, Dejan R conducted thorough reviews and critiques to ensure the accuracy and quality of the manuscript.. All authors have read and agreed to the published version of the manuscript.
Funding: N/A
Ethical Statement: This meta-analysis and systematic review is not engaged with conflict of interest nor has a source of funding. To improve the process of transparency adhering to the highest standards of quality, the research utilized the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) guidelines.
Conflicts of Interest: The authors declare no conflicts of interest.
References:
Aragone?s JM, Altimiras J, Alonso F, Celedo?n G, Alfonso S, Roura P et al. Incidence and clinical characteristics of Guillain-Barre? syndrome in Osona (Barcelona, Spain), 2003-2016. Neurologi?a (English Edition) 2021; 36: 525–530. DOI: https://doi.org/10.1016/ j.nrleng.2018.03.020. [Google Scholar] [Pubmed]
Bragazzi NL, Kolahi A-A, Nejadghaderi SA, Lochner P, Brigo F, Naldi A et al. Global, regional, and national burden of Guillain–Barre? syndrome and its underlying causes from 1990 to 2019. Journal of Neuroinflammation 2021; 18. DOI: https://10.1186/s12974-021-02319-4. [Google Scholar] [Pubmed]
Nguyen TP, Taylor RS. Guillain-Barre Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing 2023; Available from: https:// www.ncbi.nlm.nih.gov/books/NBK532254/ [Google Scholar] [Pubmed]
Shahar E. Current Therapeutic Options in Severe Guillain-Barre?? Syndrome. Clinical Neuropharmacology 2006; 29: 45–51. DOI: https://10.1097/00002826-200601000-00011 [Google Scholar] [Pubmed]
Zaki HA, Iftikhar H, Najam M, Masood M, Al-Marri NDR, Elgassim MAM et al. Plasma exchange (PE) versus intravenous immunoglobulin (IVIG) for the treatment of Guillain-Barre? syndrome (GBS) in patients with severe symptoms: A systematic review and meta-analysis. eNeurologicalSci 2023; 31: 100468., DOI: https://doi.org/10.1016/j.ensci.2023.10046 [Google Scholar] [Pubmed]
Diener HC and Putzki N (eds.) for the German Society for Neurology. Guidelines for Diagnosis and Therapy in Neurology (Leitlinien fu?r die Diagnostik und Therapie in der Neurologie). Georg Thieme Publishers, Stuttgart 2008 and www.dgb.de. [Google Scholar] [Pubmed]
Wollinsky KH, Hu?lser P –J., Brinkmeier H, Aulkemeyer P, Bo?ssenecker W, Huber–Hartmann K –H. et al. CSF filtration is an effective treatment of Guillain–Barre? syndrome. Neurology 2001; 57: 774–780. DOI: https://10.1212/wnl.57.5.774. [Google Scholar] [Pubmed]
Rosenow F, Haupt WF, Grieb P, Jime?nez-Klingberg C, Borberg H. Plasma exchange and selective adsorption in Guillain-Barre? syndrome —a comparison of therapies by clinical course and side effects. Transfusion Science 1993; 14: 13–15. DOI: https://10.1016/0955-3886(93)90047-X. [Google Scholar] [Pubmed]
Morosetti M, Meloni C, Gallucci MT, Rossini PM, Felicioni R, Palombo G et al. Plasmapheresis Versus Plasma Perfusion in Acute Guillain-Barre? Syndrome. ASAIO Journal 1994; 40: M638–M642. DOI: https://10.1097/00002480-199407000-00076. [Google Scholar] [Pubmed]
Haupt WF, Birkmann C, van der Ven C, Pawlik G. Apheresis and Selective Adsorption Plus Immunoglobulin Treatment in Guillain?Barre? Syndrome. Therapeutic Apheresis 2000; 4: 198–200. DOI: https://10.1046/j.1526-0968.2000.00182.x. [Google Scholar] [Pubmed]
Boedecker SC, Luessi F, Engel S, Kraus D, Klimpke P, Holtz S et al. Immunoadsorption and plasma exchange—Efficient treatment options for neurological autoimmune diseases. Journal of Clinical Apheresis 2021; 37: 70–81. DOI: https://10.1002/jca.21953. [Google Scholar] [Pubmed]
Diener H-C, Haupt WF, Kloss TM, Rosenow F, Philipp T, Koeppen S et al. A Preliminary, Randomized, Multicenter Study Comparing Intravenous Immunoglobulin, Plasma Exchange, and Immune Adsorption in Guillain-Barre? Syndrome. European Neurology 2001; 46: 107–109. DOI: https://10.1159/000050777. [Google Scholar] [Pubmed]
Klingel R, Heibges A, Fassbender C. Plasma exchange and immunoadsorption for autoimmune neurologic diseases – current guidelines and future perspectives. Atherosclerosis Supplements 2009; 10: 129–132. DOI: https://10.1016/S1567-5688(09)71827-6. [Google Scholar] [Pubmed]