Full HTML
Cognitive Impairment of Temporal Lobe Epilepsy and Risk Factors - Volume 1 Issue 1 , - (6 Months )
Pages: 17-25
Category: Original Research
Published Date: 16-05-2024
Ulziizaya Sodov1*, Tovuudorj Avirmed1, Khishigsuren Zuunnast1
Author Affiliation:
School of Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia.
Keywords:
Cognitive Impairment, Memory Loss, Seizure Frequency
Full Text:
Abstract:
Introduction: Focal epilepsy accounts for 60% of all clinical epilepsy cases, while temporal lobe epilepsy (TLE) accounts for 40%. Cognitive impairment such as language, attention, executive function, and memory impairment are common in temporal lobe epilepsy, and researchers believe that seizure frequency and duration can cause severe hippocampal sclerosis, as well as a secondary impact on neuronal metabolism and structure, which leads to cognitive impairment.
Aim: The goal is to determine the cognitive impairment of temporal lobe epilepsy, the clinical characteristics and risk factors.
Materials and Methods: A cross-sectional study of 50 adults with temporal lobe epilepsy from the National Center for Mental Health used a questionnaire and Montreal Cognitive Assessment (MoCA).
Results: We included 50 adults aged 26 to 61 with temporal lobe epilepsy, 52% of respondents were male, 48% were female, with an average age of 43.78±8.20 years. The study found that 92% of individuals showed cognitive impairment, with mean MoCA scores of 17.50±4.57. Long lasting seizure is a high-risk factor for cognitive function, with statistical significance (p=0.03). Seizure onset age was linked with increased attention impairments and poor visuospatial function (p=0.005), while higher seizure frequency was associated with decreased calculation (p=0.04), language (p=0.009), and drawing skills (p=0.013).
Conclusion: Our study found that 58% of respondents showed moderate cognitive impairment. Low education level, earlier age of seizure onset, high seizure frequency, extended seizure duration, presence of aura, and refractory temporal lobe epilepsy all have a statistically significant effect on cognitive impairment.
1. Introduction
Epilepsy is one of the most common and widespread neurological disorders, and there are more than 65 million people living with epilepsy in the world [1]. According to the World Health Organization, 80% of all people with epilepsy live in low and middle-income countries, and approximately 75% of them do not receive appropriate treatment. An average of 5,000 people is diagnosed with epilepsy per year, with an annual incidence of 49 per 100,000 in developed countries and 139 per 100,000 in low and middle-income countries (WHO). In a systematic review and meta-analysis, the incidence of epilepsy was 61.4 (95% CI 50.7–74.4) per 100,000 person-years [2]. The higher incidence in low- and middle-income countries than in high-income countries is due to the higher prevalence and risk of traumatic brain injury and CNS infections in low- and middle-income countries. Unfortunately, there are few epidemiological studies in TLE. Semah et al. [3] published a very important study where 2,200 patients with epilepsy attending a tertiary care center were classified according the criteria of the ILAE. In this study, 1369 patients (62.2%) had localization-related epilepsy. From these cases, 66% had TLE, 24% of the cases frontal epilepsy, 2% parietal, 3% occipital, and 3% multilobar [3].
Temporal lobe epilepsy (TLE) is the most prevalent type of partial epilepsy in adults [4], and cognitive impairment is a common comorbidity in temporal lobe epilepsy (TLE) and is often considered more detrimental to quality of life than seizures [5]. Temporal lobe epilepsy occurs in 20% of all epilepsy forms and 40-66% of adults with partial epilepsy, 30-40% are refractory, and two- thirds of them are treated with surgery [6] [7].
Recently, several studies have shown that recurrent seizures affect all aspects of cognitive functioning including attention, language, praxis, executive function (intelligence), judgment, insight, and problem solving [8]. Long-term epilepsy research has shown that uncontrolled, chronic, severe seizures cause memory loss in 17-38% of individuals with episodes lasting 2-10 years [9]. The left hemisphere of the brain plays a major role in memory function, and in a comparative study of right and left hemisphere epilepsy with hippocampal sclerosis, patients with left hemisphere epilepsy had lower score on the verbal memory test [10].
Seizure frequency and duration can cause severe hippocampal sclerosis, as well as a secondary impact on neuronal metabolism and structure, which leads to cognitive impairment [11]. Many factors contribute to cognitive impairment in epilepsy which includes the main etiology of epilepsy, localization (mesial temporal lobe), lateralization (right or left hemispheres), factors associated with seizures (age at seizure onset, seizure frequency, duration of seizure, multiple episodes of status epilepticus), and treatment (number of anti-seizure medications, epilepsy surgery) [12].
TLE is often unresponsive to anti-seizure treatment, with half of patients progressing to refractory epilepsy. Increased frequency of seizure is not only a medical problem, but also a risk factor for cognitive impairment.
2. Material and Methods
The cross-sectional study included patients with epilepsy under follow-up at the National Center for Mental Health from 2021 to 2022. First, the medical history of 460 patients under control was studied and seizure-related information, such as clinical features, also epileptic changes of Electroencephalography (EEG) were sampled. After the initial sampling, 87 patients were selected based on the clinical manifestations of seizures and EEG changes that could be indicative of temporal lobe epilepsy.
2.1 Participants
According to the exclusion criteria, 50 patients were included in this study and 37 cases excluded. In the questionnaire section included demographics, socioeconomic information, other disease, and seizure related information. Seizures related questionnaire includes writing hand, family history of seizures, age at seizures onset, cause of seizures, clinical forms of seizures, duration and frequency of seizures, status epilepticus, current anti-seizure medication dose and number, and symptoms of cognitive decline. Questionnaire to assess the clinical characteristics of seizures included presence of aura, type of aura, post-ictal awareness, state of consciousness, post-ictal automatism, and aphasia.
We used the Montreal Cognitive Assessment (MoCA) to determine cognitive impairment. The Montreal Cognitive Assessment (MoCA) is a brief cognitive screening tool with high specificity (87%) and sensitivity (90%) for detecting mild cognitive impairment. Executive function, language abilities, and visuospatial processing are assessed more rigorously with the MoCA relative to the MMSE [13]. In recent years, the MoCA has been used to assess cognitive impairment in many diseases, including neurological disorders, brain tumors, multiple sclerosis, sleep disorders, depression, and schizophrenia. It is allowed to use according to the unique characteristics, language and culture of the country or nation. According to the score, cognitive impairment was evaluated as mild (18-25), moderate (10-17), severe (below 10), and a score above 26 was considered normal.
2.2 Study Ethics
Ethical permission to start the research was obtained from the Medical Ethics Review Committee of Mongolian National University of Medical Science (MNUMS). In accordance with the principles of research ethics, the client and their guardians were informed about the purpose of the study, the principles of conducting the study, the confidentiality of information, and the results of the study, and consent was obtained. Surnames and personal information of the research participants were kept secret by encoding numbers from 01 to 50.
2.3 Data Analyses
Analyses were conducted using SPSS 26 statistical analyzing program. The results of summary analysis were expressed as mean (standard deviation) and percentage (number), and standard deviation of the mean variation of numerical values. Statistical analyses adjusted with risk factors and confounding factors and chi square, binary regression analysis, Fisher Exact, Independent T test was used. Also, the OR, its 95% confidence interval, and statistical significance at the 0.05 level were calculated.
3. Results
3.1 Characteristics
Twenty-four (48%) female and 26 (52%) male patients with TLE participated in the study. The mean age of participants was 43.7 ± 8.2 (range, 26–61) years. The mean age of seizure onset was 15.0 ± 9.2 (range, 1–50) years and mean duration of seizure was 28.8 ± 8.5 years. Demographic data and other characteristics of cases are summarized [Table 1].
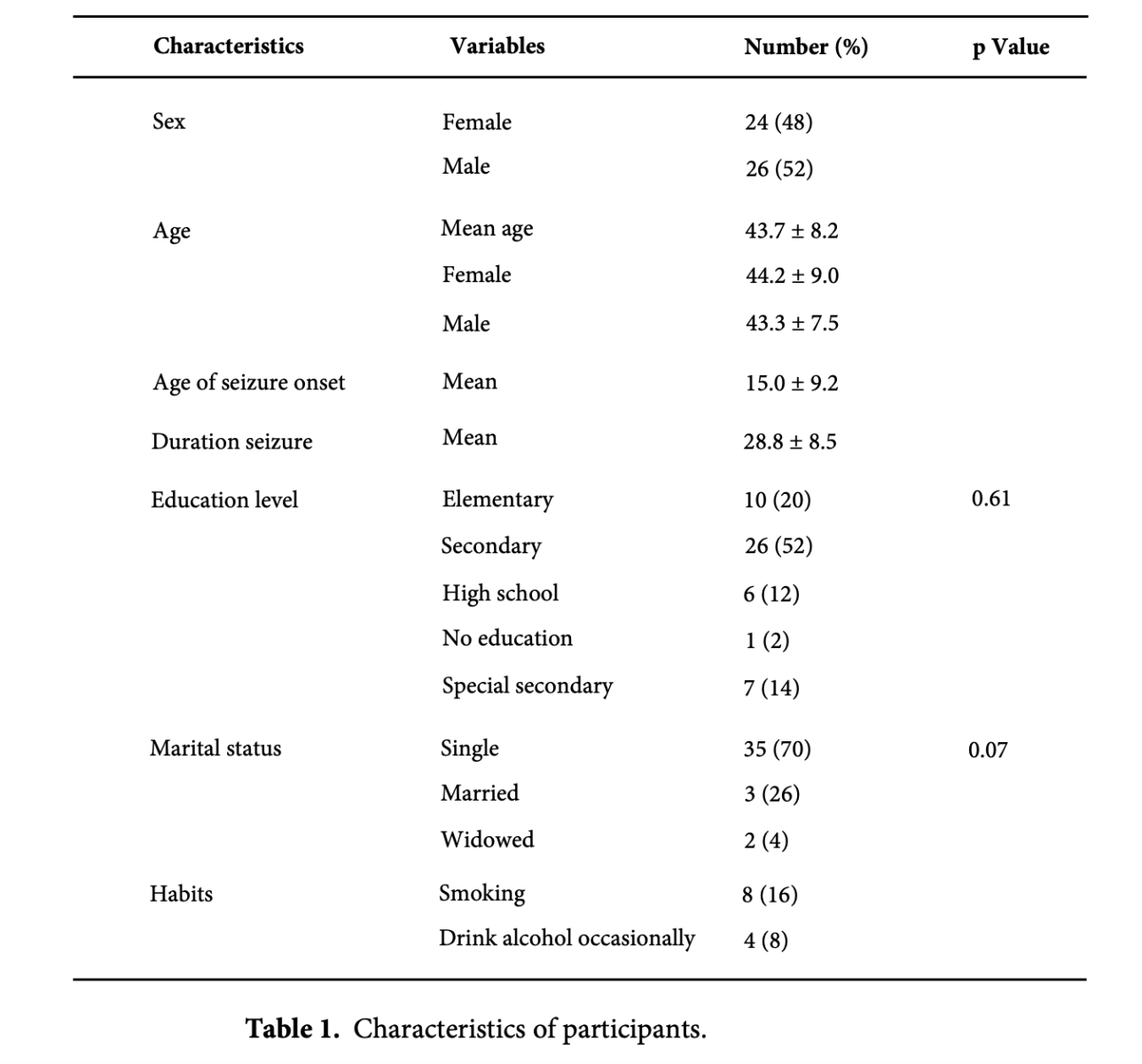
There were no significant differences among the patients in terms of age, gender, or educational level. The mean duration from seizure beginning to psychiatric monitoring was 9.2 ± 6.6 years, with disease-related impairment occurring at an average age of 24.1 ± 8.2 years. People with epilepsy have been disabled for an average of 19.6 years, and they have lost the ability to study and live a normal life.
In terms of education level, 66% of the participants (n = 33) received secondary or special secondary education, and there was no gender difference in education level (p = 0.61). In our study, seizure start ages ranged from one to fifty years, and 70% of the individuals were unmarried and single. The group with early-onset seizure had a higher likelihood of being single or unmarried (p = 0.003).
3.2 3.2 Cognitive impairment and risk factors in TLE
According to the questionnaire, 33 (66%) of the participants reported memory loss, 26 (52%) reported loss of concentration, 12 (32%) reported decreased writing and drawing skills, 15 (30%) had speech and language problems, 10 (20%) had long-term memory, and 4 (8%) were lacking in visuospatial and orientation skills (Table 2).
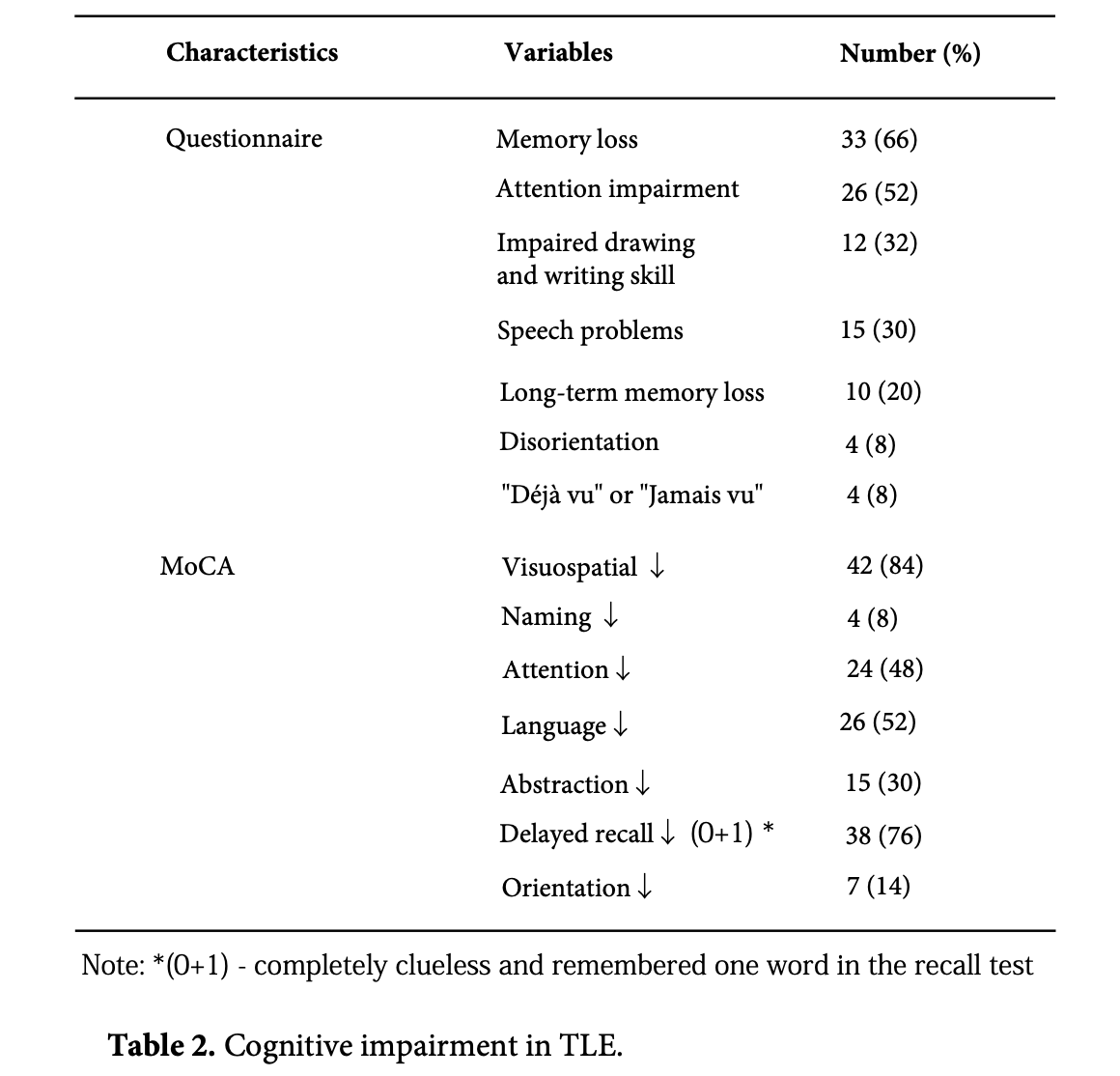
According to the MoCA score, 92% of the study participants had cognitive impairment, 34% (17) had mild cognitive impairment, and 58% (29) had moderate cognitive impairment. In total, 84% of the study participants had reduced visual processing, 76% had reduced memory, 52% had reduced language skills and vocabulary, and 48% had reduced attention. The mean MoCA score was 17.50 ± 4.57, 16.62 ± 4.11 for men, and 18.46 ± 4.93 for women, and no gender difference was observed for cognitive test scores. The mean score for seizures lasting more than 16 years was 17.15 ± 4.41, while for seizures lasting 11-15 years, it was 25.00 ± 2.82. Long-term seizures have a statistically significant effect on cognitive performance (p=0.03). A significant link was found when assessing whether our study participants' relatively low level of education (12% had higher education) was associated with increased impairment in the visuospatial domain (p=0.04). 56% of participants failed to draw the cube, while just 30% passed the clock drawing challenge. Furthermore, 52% failed the calculation test, and it was statistically significant that low educational level was a factor influencing attention deficit (p = 0.02) (Table 3).
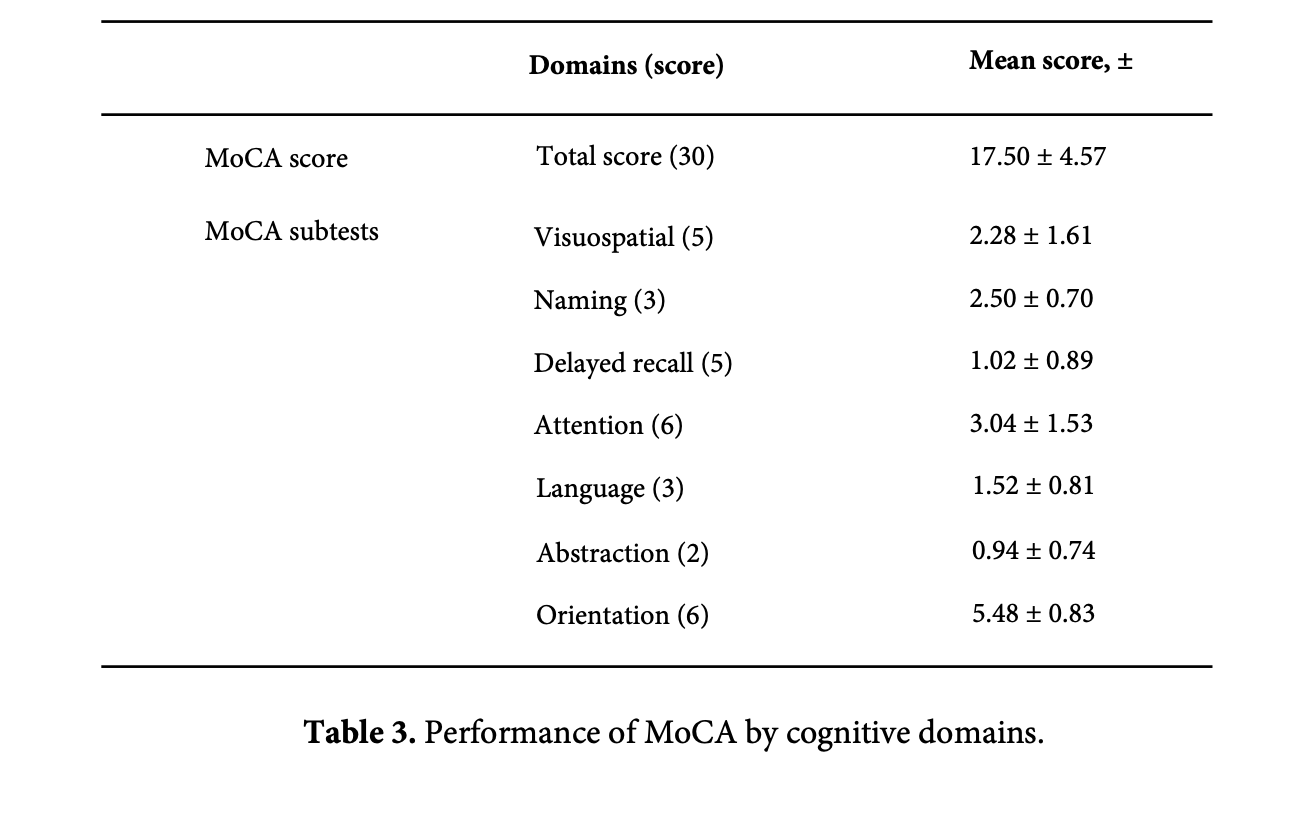
Early-onset seizures are more likely to cause deficiencies in attention, visuospatial, and executive skills (p = 0.005). Attention deficit was more prevalent in the group with younger seizure onset, and the results were statistically significant (p = 0.001, CI 95% 15.0-21.8). On the other hand, higher seizure frequency has an effect on calculating (p = 0.04), speaking (p = 0.009), and drawing skills (p = 0.013). In the naming tests, 88% (44) of the participants failed, while 44% failed the phrase repetition test, indicating that people with temporal lobe epilepsy had clearly lowered language abilities. Seizures, whether early-onset or chronic and progressive, affect language and vocabulary skills.
The higher the frequency of seizure is related to the lower the cognitive test score, which is statistically significant (p<0.005). In addition, having refractory TLE was more likely to lead to cognitive decline (p=0.03). However, when this result was adjusted for confounding factors such as frequency of seizure and level of education and tested by Fisher's test, the statistical significance is not confirmed (p=0.056). This is considered to be related to our study's small sample size of eight out of fifty individuals with refractory TLE (Table 4).
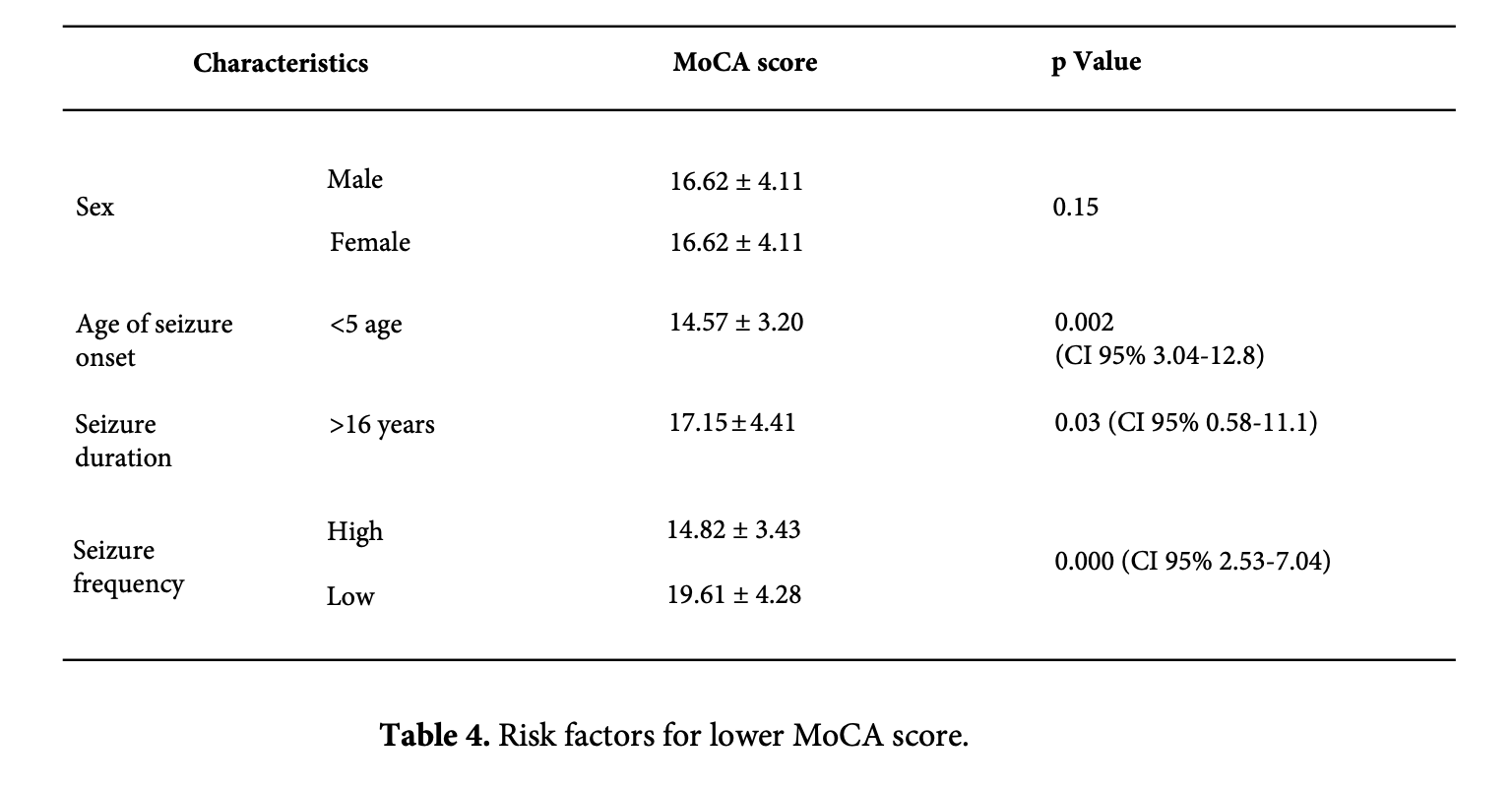
It is statistically likely that the decline in MoCA test is influenced by the early-onset seizure, duration of seizure, and the seizure frequency (Table 4). Seizures occurring under the age of 5 are associated with decreased orientation, attention, and drawing skills (p=0.001, 0.003, and 0.01, respectively). A higher frequency of seizure affects calculating (p=0.04), language (p=0.009) and drawing skills (p=0.013). Seizures occurring before the age of five are associated with poorer orientation, attention, and drawing skills (p=0.001, 0.003, and 0.01, respectively). A higher frequency of seizures has an impact on calculating (p=0.04), language (p=0.009), and drawing skills (p=0.013).
The presence of aura was found to be linked with cognitive decline, and statistical regression (binary) revealed that aura was more common in the group with mild cognitive impairment (p=0.03). There was no significant link found between the presence of automatism and cognitive test scores.
4. Discussion
Epilepsy is a chronic neuropsychiatric disease manifested by seizure syndrome, and it occurs regardless of age and gender depending on the cause of the disease. Our study included 50 adults with TLE, average age was 43.78 ± 8.20 years, 52% were male, 48% were female, and no statistically significant correlation was observed for gender. Recently, several studies have shown that the age atseizure onset has an effect on the clinical feature of temporal lobe epilepsy, seizure onset tends to be relatively younger in TLE [14-16]. In our study, the mean age of seizure onset in the group with TLE was 15.02 ± 9.28 years, while the mean age of seizure onset in the other epilepsy case group (n=337) was 19.1 ± 21.1 years. Young seizure-onset, long disease duration, and the high frequency of seizures causes psychological and behavioral changes, as well as cognitive deterioration during epilepsy, which reduces quality of life. Our study found that the average period from seizure
beginning to psychiatric supervision was 9.28 ± 6.61 years, while the average age of disease- related disability was 24.18 ± 8.23 years.
People with epilepsy spent an average of 19.6 years with disability, lost the opportunity to study and live a quality life. This may be due to the small sample size of our study, in which Singh et al. (2020) published that people with epilepsy in low-income countries had an average length of time living with disability of 43.44 years, which is higher than ours. However, in developed countries, the average life expectancy with disability is 8.4 years, which reflects the social and economic status of the country, the health education of citizens, and the quality of comprehensive health care services, including epilepsy care [17].
In our study, the average age of seizure onset was 15.02 ± 9.28 years and the average duration of seizure was 28.88 ± 8.55 years, while in the study by Szaflarski (2006), the average age of seizure onset was 19 ± 15 years and the average duration of seizure was 18 ± 16 years. It is believed that the later-onset seizure, the shorter duration of seizure and the longer the health-related quality of life is maintained [18]. These results suggest that the mean age of seizure onset is similar, but the mean years of seizure duration in our study are longer, which may be influenced by epilepsy treatment, access to anti-seizure medication, and choice of medications.
Education levels for people with epilepsy vary by country, however most people have low level of education and depending on the country's development and social status. A low degree of education suggests a low brain capacity, whereas more education correlates with higher cognitive ability and greater resistance to cognitive decline with neurological function loss [19].
Sixty-six (66%) of our respondents had secondary education, 20% had elementary education, and 12% had higher education. Oyegbile et al. (2004) included 96 patients with TLE found that those with higher level of education had a different relationship between epilepsy duration and cognitive decline than those with lower level of education [20]. In our study, lower educational attainment was statistically significantly associated with lower MoCA scores (p=0.01). In our study, the mean age was 43.78 ± 8.20 years, the mean age of seizure onset was 15.02 ± 9.28, and the mean MoCA score was 17.50 ± 4.57, respectively. These results are consistent with Yang et al. (2018) included 40 patients with TLE between the ages of 13-43, with a mean age of 26.88 ± 7.6, age of seizure onset was 14.37 ± 7.1, and a mean MoCA score of 20.8 ± 3.2 and in accordance with the relatively higher age in our study, it is believed that the number of years of seizures is longer and the average MoCA score is slightly lower [21]. Also, Montan?o-Lozada et al. (2021) evaluating the cognitive decline of patients with TLE in outpatients, the mean age was 30.73 ± 10.54 years, the mean MoCA score 19.62 ± 5.83, and the educational level was correlated with the low score of the MoCA test (p=0.001) is similar with our study results (p=0.01) [22]. Souza et al. (2021) reported that mean MoCA score was 17.2 ± 5.4, the mean age 44.7, and the mean MoCA score in the group with a low education level was 14.6 ± 4.7, which is similar to our results [23].
Several studies of cognitive impairment in TLE [19,24,25] have reported significant impairment in naming, language, delayed recall, and concentration. In our study, 88% of participants failed with naming test and 82% failed the attention test, indicating a significant impairment of language, vocabulary, concentration, and memory in people with TLE. Impaired language and speech skills are frequent in people with TLE, especially dominant hemisphere epilepsy [26] and the risk increases as early-onset seizures [27,28]. Attention deficits and executive function deficits are common in people with TLE, and early-onset seizure is the strongest risk factor. In our study, it was observed that the visuospatial, orientation, attention, and drawing skills impaired in the group with under 5 years of age seizure onset (p=0.001, p=0.003, p=0.01).
Cognitive decline in TLE may be related to the duration of epilepsy [29], as well as the total number of seizures over a lifetime [30]. In our study, the mean MoCA score was 17.15 ± 4.41 in the group with a seizure duration more than 16 years and it was statistically significant (p=0.03) that long duration of seizures is a risk factor affecting cognitive function.
In addition, postictal automatism was common in TLE, and there were 3 patients who committed socially dangerous behavior due to postictal loss of consciousness, which is a noteworthy direction for further study.
5. Limitations
According to a review of the literature, memory loss is more common cognitive impairment in temporal lobe epilepsy. According to the results of our research, 76% of the participants were found to have memory loss, but there were no statistically significant results in risk factors affecting it. We believe that this may be due to the fact that we used a single test method to assess cognitive impairment in our study.
Our study found that 58% of respondents showed moderate cognitive impairment, with an average MoCA score of 17.50 ± 4.57. Cognitive domains such as visuospatial function, memory, language skills, vocabulary, and attention were compromised. Low education level, earlier age of seizure onset, high seizure frequency, extended seizure duration, presence of aura, and refractory temporal lobe epilepsy all have a statistically significant effect on cognitive impairment.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors thank for the National Center for Mental Health for their deep support in conducting this study and professors of the Department of Psychiatry and Neurology of MNUMS.
Highlights
- TLE is often unresponsive to anti-seizure treatment, with half of patients progressing to refractory epilepsy. Increased frequency of seizure is not only a medical problem, but also a risk factor for cognitive impairment.
- The higher the frequency of seizure, long-term seizure is related to the lower the cognitive test score, which is statistically significant.
- According to the MoCA score, 34% (17) had mild cognitive impairment, and 58% (29) had moderate cognitive impairment and the mean MoCA score was 17.50 ± 4.57.
- Level of education was a protective factor for cognitive function, and lower level of education was statistically significantly associated with lower MoCA scores.
- In addition, having refractory TLE was more likely to lead to cognitive decline.
References:
Merkena MD. Prevalence of Cognitive Adverse Outcomes in Epileptic Outpatients. J Neurol Stroke. 2016;4:155–156. DOI: 10.15406/ jnsk.2016.04.00155 [Google Scholar][Pubmed]
Gill SJ, Lukmanji S, Fiest KM, Patten SB, Wiebe S, et al. Depression screening tools in persons with epilepsy: A systematic review of validated tools. Epilepsia. 2017;58(5):695–705. DOI: https://doi.org/10.1111/epi.13651 [Google Scholar][Pubmed]
Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence. Neurology. 1998;51(5):1256–1262. DOI: https://doi.org/10.1212/wnl.51.5.1256 [Google Scholar][Pubmed]
Téllez-Zenteno JF, Hernández-Ronquillo L. A Review of the Epidemiology of Temporal Lobe Epilepsy. Epilepsy Res Treat. 2012:1–5. DOI: 10.1155/2012/630853 [Google Scholar][Pubmed]
Titiz AS, Mahoney JM, Testorf ME, Holmes GL, Scott RC. Cognitive impairment in temporal lobe epilepsy: Role of online and offline processing of single cell information. Hippocampus. 2014;24(9):1129–1145. DOI: https://doi.org/10.1002/hipo.22297 [Google Scholar] [Pubmed]
Kim J, Kim SH, Lim SC, Kim W, Shon YM. Clinical characteristics of patients with benign nonlesional temporal lobe epilepsy.
Neuropsychiatr Dis Treat. 2016;12:1887–1891. DOI: https://doi.org/10.2147/ndt.s110400 [Google Scholar][Pubmed]
Thom M, Mathern GW, Cross JH, Bertram EH. Mesial temporal lobe epilepsy: How do we improve surgical outcome. Ann Neurol.
2010;68(4):424–434. DOI: https://doi.org/10.1002%2Fana.22142 [Google Scholar][Pubmed]
Zhao F, Kang H, You L, Rastogi P, Venkatesh D, et al. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Ann Indian Acad Neurol. 2014;17(4):374–382. DOI: https://doi.org/10.4103/0972-2327.144003 [Google Scholar][Pubmed]
Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–453. DOI: https:// doi.org/10.1016/s0079-6123(02)35041-6 [Google Scholar][Pubmed]
Phuong TH, Houot M, Méré M, Denos M, Samson S, Dupont S. Cognitive impairment in temporal lobe epilepsy: Contributions of lesion, localization and lateralization. J Neurol. 2021;268(4):1443–1452. doi:10.1007/s00415-020-10307-6. DOI: https://doi.org/10.1007/
s00415-020-10307-6 [Google Scholar][Pubmed]
Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: A cross sectional study. J Neurol Neurosurg Psychiatry. 1999;67(1):44–50. DOI: https://doi.org/10.1136%2Fjnnp.67.1.44 [Google Scholar][Pubmed]
Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(2):96–98. DOI: https://doi.org/10.1111/j.1528-1167.2006.00702.x [Google Scholar][Pubmed]
Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening tool for Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53(4):695–699. DOI: https://doi.org/10.1111/j.1532-5415.2005.53221.x [Google Scholar][Pubmed]
Foldvary N, Lee N, Thwaites G. Clinical and electrographic manifestations of lesional neocortical temporal lobe epilepsy. Neurology.
1997;49(3):757–768. DOI: https://doi.org/10.1212/wnl.49.3.757 [Google Scholar][Pubmed]
Villanueva V, Serratosa JM. Temporal lobe epilepsy: Clinical semiology and age at onset. Epileptic disorders. 2005;7(2):83–90. DOI: https:// doi.org/10.1684/j.1950-6945.2005.tb00107.x [Google Scholar][Pubmed]
Reyes A, Kaestner E, Ferguson L, Jones JE, Seidenberg M, et al. Cognitive phenotypes in temporal lobe epilepsy utilizing data- and clinically driven approaches: Moving toward a new taxonomy. Epilepsia. 2020;61(6):1211–1220. DOI: https://doi.org/10.1111/epi.16528 [Google Scholar][Pubmed]
Singh G, Sander JW. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy and Behavior.
2020;105(Apr):1–3. DOI: https://doi.org/10.1016/j.yebeh.2020.106949 [Google Scholar][Pubmed]
Szaflarski M, Meckler JM, Privitera MD, Szaflarski JP. Quality of life in medication-resistant epilepsy: The effects of patient’s age, age at seizure onset, and disease duration. Epilepsy and Behavior. 2006;8(3):547–551. DOI: https://doi.org/10.1016/j.yebeh.2006.01.001 [Google Scholar][Pubmed]
Marques CM, Caboclo LO, da Silva TI, Noffs MH, Carrete H J. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy and Behavior. 2007;10(3):477–485. DOI: https://doi.org/10.1016/j.yebeh.2007.02.002 [Google Scholar][Pubmed]
Oyegbile TO. The natural course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;60(10):1736–1742. DOI: https://doi.org/10.1212/01.wnl.0000125186.04867.34 [Google Scholar][Pubmed]
Yang H, Zhang C, Liu C, Yu T, Zhang G, et al. Brain network alteration in patients with temporal lobe epilepsy with cognitive impairment.
Epilepsy and Behavior. 2018;81(Apr):41–48. DOI: https://doi.org/10.1016/j.yebeh.2018.01.024 [Google Scholar][Pubmed]
Montaño-Lozada JM, López N, Espejo-Zapata LM, Soto-Añari M, Ramos-Henderson M et al. Cognitive changes in patients with epilepsy identified through the MoCA test during neurology outpatient consultation. Epilepsy & Behavior. 2021;122:108158. DOI: https:// doi.org/10.1016/j.yebeh.2021.108158 [Google Scholar][Pubmed]
De Souza MC, De Paulo CO, Miyashiro L, Twardowschy CA. Comparison of screening tests in the evaluation of cognitive status of patients with epilepsy. Dementia e Neuropsychologia. 2021;15(1):145–152. DOI: https://doi.org/10.1590/1980-57642021dn15-010016
[Google Scholar][Pubmed]
Black LC, Schefft BK, Howe SR, et al. The effect of seizures on working memory and executive functioning performance. Epilepsy Behav.
2010;17(3):419–420. DOI: https://doi.org/10.1016/j.yebeh.2010.01.006 [Google Scholar][Pubmed]
Nouha F, Sawsan D, Salma S, Olfa H, Hanen HK, et al. Cognitive Impairment in Patients with Temporal Lobe Epilepsy. J Neuropsychiatr.
2018;2(5):1–7. DOI: https://doi.org/ 10.21767/2471-8548.10009 [Google Scholar][Pubmed]
Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuro Image. 2007;36(1):209–221. DOI: https://doi.org/10.1016/j.neuroimage.2007.02.028 [Google Scholar][Pubmed]
Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided Interictal Epileptic Activity Induces Shift of Language Lateralization in Temporal Lobe Epilepsy: MRI Study. Epilepsia. 2006;47(5):921–927. DOI: https://doi.org/10.1111/j.1528-1167.2006.00514.x [Google Scholar][Pubmed]
Dijkstra KK, Ferrier CH. Patterns and predictors of atypical language representation in epilepsy. J Neurol Neurosurg Psychiatry 2013;84(4):379–385. DOI: https://doi.org/10.1136/jnnp-2012-303141 [Google Scholar][Pubmed]
Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog Brain Res. 2002;135(2):429–438. DOI: https://doi.org/10.1046/1528-1157.2002.49901.x [Google Scholar][Pubmed]
Dodrill CB. Progressive cognitive decline in adolescents and adults with epilepsy. Prog Brain Res. 2002;135(2):399–407. DOI: https:// doi.org/10.1016/s0079-6123(02)35037-4 [Google Scholar][Pubmed]